
The idea that the evolution of a system can change as a result of a measurement is one of the topics that is currently debated among quantum theorists. This discontinuous change in the quantum state of the system as a result of the measurement is known as the collapse of the wavefunction.

Consequently, any subsequent measurement of the energy would yield the value \(E_1\) with 100% certainty. Because spectroscopic time-dependent perturbations are extremely common in chemistry, we will focus much of our attention to this class of perturbations in this Chapter.
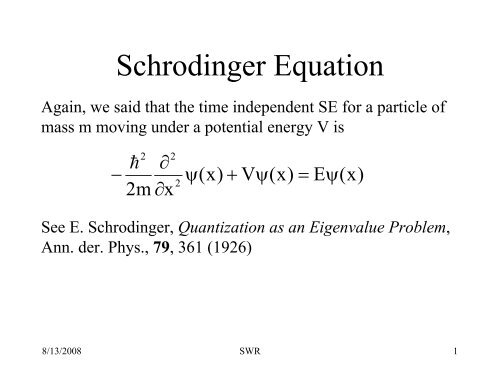
equation: they allow for a natural, physical interpretation on the basis of Huygens. Hence, just after the measurement, the state must be \(\psi_1 (x)\), which means that because of the measurement, any further dependence on \(\psi_2 (x)\) drops out, and for all time thereafter, there is no dependence on \(\psi_2 (x)\). time-dependent propagator for quantum systems with time-independent. From the above discussion, there is only one possibility for the state of the system, and that has to be the wavefunction \(\psi_1 (x)\), since in this state we know with 100% certainty that the energy is \(E_1\). What is the state of the system just after the measurement is made? Once we make the measurement, then we know with 100% certainty that the energy is \(E_1\). Now, at some specific instance in time \(t\), we measure the energy and obtain a value \(E_1\). Now equation (7) can be written as: Schrodinger time independent wave equation. The factor H is known as a Hamiltonian operator which gives the energy of the particle. At any point in time, the state \(\Psi (x,t)\) will be some mixture of \(\psi_1 (x)\) and \(\psi_2 (x)\), and this mixture changes with time. The total energy of the particle is the sum of potential energy (P.E) and kinetic energy (K.E) which is given by: This is the Schrodinger time-dependent wave equation formula.


 0 kommentar(er)
0 kommentar(er)
